The device is expected to be available for purchase this fall. It is battery powered and can be life saving. With the Michael Jackson’s loss of life due to cardiac arrest, no doubt devices as such might be more in the forefront. The device has been approved in both Europe and Canada as well.
The device can be used in hospitals and cath labs. More information with additional images here. Emergency vehicles such as ambulances of course are right up there on the list. The device can be more consistent than a human can manually. BD
Press release:
FDA Grants LUCAS™ 2 Chest Compression System 510(k) Clearance in the United States
Next-Generation Product is Electric, Making It the Lightest, Most Compact Device Available for Mechanical Chest Compressions
REDMOND, WASH. / LUND, SWEDEN – June 29, 2009 – Physio-Control Inc., a wholly-owned subsidiary of Medtronic, Inc. (NYSE: MDT), announced today that LUCAS™ 2, the next-generation LUCAS™ Chest Compression System, has been granted 510(k) market clearance by the U.S. Food and Drug Administration (FDA). Developed and manufactured by Jolife AB and distributed exclusively in the United States by Physio-Control, the LUCAS 2 is an automated, battery-powered device that is designed to give consistent, uninterrupted compressions to victims in cardiac arrest.
Since performing manual chest compressions according to the American Heart Association guidelines is both difficult and physically demanding, Jolife created the LUCAS Chest Compression System to assist. LUCAS is designed to deliver uninterrupted compressions at a consistent rate and depth (100 compressions per minute, depth 1.5 to 2 inches) to facilitate the return of spontaneous circulation in cardiac arrest patients. By ensuring consistent blood flow, LUCAS helps emergency medical responders deliver a more viable candidate for recovery to the Emergency Department. Furthermore, LUCAS can assist in improving the operations of an emergency response system or hospital by helping to reduce the chaos on the scene and free up staff for other emergencies.
LUCAS 2 builds upon the well-proven LUCAS™ 1 technology, but differs from its predecessor in that it is an electric rather than a pneumatic device. LUCAS 2 can be powered either by battery alone or using a wall or car electricity outlet. The battery is the latest in rechargeable, Lithium Ion Polymer technology and operates for up to 45 minutes (typical) on a single battery. LUCAS 2 is equipped with Smart Restart functionality. When a battery needs to be replaced, LUCAS 2 does not have to be powered down, only put into the pause mode, and when the new battery is inserted, the start position will stay the same within 60 seconds from the pause. Operation can be quickly resumed, saving time for medical personnel. LUCAS 2 offers other new features such as alerts and pauses to aid ventilation during compressions with an unsecured airway (e.g. bag-valve-mask) and quieter operation. 
“With the LUCAS 2 we now offer a solution to customers that prefer a battery-powered device, and the LUCAS 1 for those who prefer the pneumatic solution,” said Erik von Schenck, CEO of Jolife AB. “The rechargeable battery requires a minimum of maintenance, and makes the device the most compact, lightweight and portable mechanical chest compression device on the market.”
LUCAS 2 weighs only 17.2 lbs (7.8kg) and fits into a compact backpack measuring just 25.6h x 13w x 19.8d inches (65h x 33w x 25d centimeters).
“We are proud that our strong partnership with Jolife has enabled us to bring another high-quality, innovative, life-saving product to the emergency care market,” said Brian Webster, president of Physio-Control. “Customers have been asking us for a battery-powered LUCAS device for a couple of years and with LUCAS 2 we are able to deliver.”
Within the past month, LUCAS 2 has also received a CE (Conformité Européenne) Mark in Europe and marketing approval from Health Canada. Physio-Control plans to begin shipping to the U.S. markets in the fall.
About LUCAS CPR
LUCAS Chest Compression System is an easy-to-use and lightweight device that provides quality chest compressions according to the European Resuscitation Council and American Heart Association Guidelines for CPR (cardiopulmonary resuscitation). It assists rescuers in facilitating the delivery of vital oxygen to the brain and priming the heart for a defibrillation shock in cardiac arrest situations. LUCAS is simple to use, applied within seconds and feasible for use on most cardiac arrest patients. LUCAS is used by firefighters and paramedics on the scene of a cardiac arrest and used by clinical personnel in the emergency departments and cath labs of hospitals. 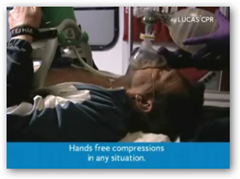
About Jolife AB
Jolife AB, founded in 2000, develops and manufactures the LUCAS Chest Compression System. Jolife works closely with leading physicians and paramedics and is committed to research and development in order to continue to offer innovative products. The LUCAS Chest Compression Systems are sold in the major markets in the world. Based in Lund in southern Sweden, Jolife markets its products through an exclusive global distribution agreement with Physio-Control, Inc., a division of Medtronic, Inc. – except in Sweden, Norway and Finland, where Jolife sells direct.
About Physio-Control
Physio-Control, a wholly-owned subsidiary of Medtronic, is located in Redmond, Wash. Physio-Control pioneered defibrillation technology more than 54 years ago. With nearly 800,000 LIFEPAK® defibrillators distributed worldwide, the company is the world’s leading provider of external defibrillation and monitoring technology for the treatment of sudden cardiac arrest and other cardio-respiratory emergencies. To find out more about Physio-Control, go to www.physio-control.com or call 1-800-442-1142.
About Medtronic
Medtronic, Inc. (www.medtronic.com), headquartered in Minneapolis, is the global leader in medical technology – alleviating pain, restoring health, and extending life for millions of people around the world.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic’s Annual Report on Form 10-K for the year ended April 24, 2009. Actual results may differ materially from anticipated results.




0 comments :
Post a Comment