It’s been a while since we heard about insulin that can be inhaled, as Pfizer abandoned their drug called Exubera, which everyone somewhat commented that it looked more like a bong. You can see from the picture here, this one does not look like a bong and is much smaller in size. Incase you missed any of the stories about it from a while back and want to compare the size, you can link here to a video.
Is it a bong or Exubera?
The jury is still out but we could still end up with insulin that is inhaled, a much easier process than injections by all means and less sticks. BD
MannKind Corp.’s stock jumped 14 percent Monday after the Valencia biotech said the U.S. Food and Drug Administration accepted and is now reviewing its application for the inhaled insulin drug Afresa.
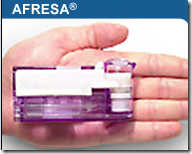
Having the regulator accept the application on the company’s first try is considered a significant milestone. Competitor Pfizer Inc. pulled a similar drug off the market for safety reasons, and other companies later dropped their own development programs.
From the Website:
Our lead investigational product candidate, AFRESA® , an ultra rapid-acting insulin, has completed phase 3 clinical trials in the United States, Canada, Europe and Latin America.
We believe that the pharmacokinetic profile of AFRESA® sets it apart from all other insulin products. AFRESA®'s particles dissolve upon contact with the lung surface, releasing insulin that rapidly enters the bloodstream. It achieves peak insulin levels within 12-14 minutes of administration, which more closely mimics the natural release of insulin than any other insulin preparation.
We have conducted an extensive clinical program for AFRESA®, involving more than 40 separate studies. Approximately 5,300 subjects participated in our clinical studies, of which more than 2,900 subjects have taken AFRESA®. Based on our clinical studies, the use of AFRESA® appears to result in the following clinical benefits compared to rapid acting insulin analogs:
Comparable A1C reductions
Superior post prandial glycemic control
Improved fasting glucose control
Less hypoglycemia due to better synchronization with meal absorption and glucose elimination
Little or no weight gain
No adverse effect on pulmonary function
In collaboration with a marketing partner, we plan to establish AFRESA® as the preferred prandial drug therapy for diabetes care.
Los Angeles Business Journal Online - business news and information for Los Angeles California



0 comments :
Post a Comment